- Valence electrons determine chemical properties.
- Atomic number indicates the number of valence electrons.
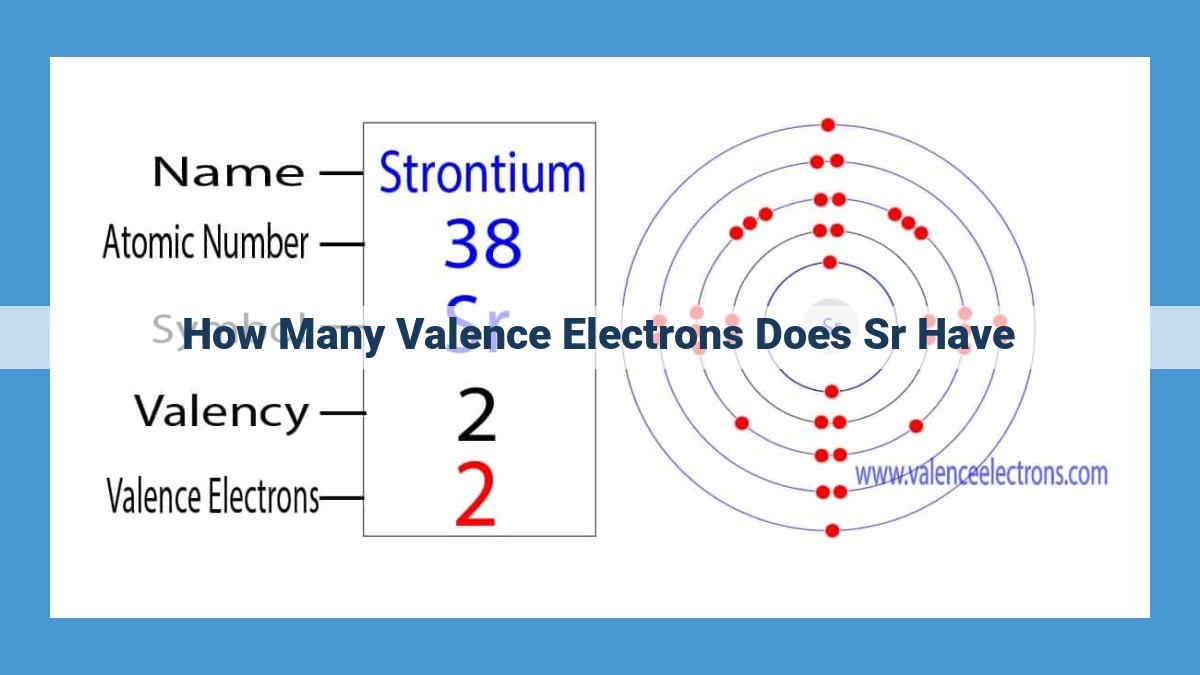
- Strontium, with an atomic number of 38, has two valence electrons in its outermost shell.
- Strontium belongs to Group 2 alkaline earth metals, which have a tendency to have two valence electrons.
- The number of valence electrons influences strontium’s reactivity and chemical behavior.
- Definition and explanation of valence electrons
- Their role in determining chemical properties
Valence Electrons: The Gateway to Chemical Understanding
In the vast and intricate world of chemistry, the concept of valence electrons holds a pivotal role. These are the electrons that reside in an atom’s outermost energy level, the valence shell, and they play a crucial part in determining an element’s chemical properties.
Unveiling the Essence of Valence Electrons
Valence electrons are akin to the social butterflies of the atomic realm. They are highly energetic and eager to interact with other atoms, forming the chemical bonds that create the molecules that make up our world. The number of valence electrons an atom possesses directly influences its chemical reactivity and bonding behavior.
Delving into the Nexus of Atomic Structure and Valence Electrons
The number of valence electrons an atom has is closely linked to its atomic number. The atomic number represents the number of protons in the atom’s nucleus, and protons carry a positive charge. Valence electrons, on the other hand, carry a negative charge. To maintain electrical neutrality, the number of valence electrons must balance the number of protons.
Electron Configuration: Mapping the Energy Landscape
Electron configuration, often represented as a string of numbers and letters, provides a detailed map of the electron distribution within an atom. It reveals the energy levels and the number of electrons occupying each level. The outermost energy level, the valence shell, houses the valence electrons.
Strontium: A Case Study
Let’s take strontium as an example to illustrate the concept of valence electrons. Strontium, with an atomic number of 38, has 38 protons and 38 electrons. To maintain electrical neutrality, strontium must have 38 valence electrons.
Unraveling Strontium’s Valence Electrons
The electron configuration of strontium can be represented as: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s². The last two electrons, in the 5s orbital, are the valence electrons. They reside in the outermost valence shell and determine strontium’s chemical behavior.
Periodic Trends: Alkaline Earth Metals
Strontium belongs to a group of elements known as alkaline earth metals. These elements are located in Group 2 of the periodic table and are characterized by having two valence electrons. This consistent valence electron count explains their similar chemical properties, such as high reactivity and a tendency to form stable divalent cations.
The number of valence electrons an atom possesses is a fundamental property that governs its chemical behavior. By understanding valence electrons, we gain a deeper insight into the interactions between atoms and the formation of molecules. This knowledge serves as a cornerstone for unraveling the complexities of chemistry and predicting the properties of materials.
Atomic Structure and Valence Electrons: A Deeper Dive
In our journey to unveil the mysteries of valence electrons, we must first delve into the fundamental structure of atoms. Each atom, the building block of matter, possesses a nucleus at its core, packed with positively charged protons and neutrally charged neutrons. Surrounding the nucleus is a swarm of electrons, negatively charged particles that dance in constant motion.
Relationship between Atomic Number and Valence Electrons
The atomic number of an element is a defining characteristic that identifies it from all others. It represents the number of protons in the atom’s nucleus, which in turn determines the number of electrons orbiting around it.
Determining Valence Electrons from Atomic Number
To understand the concept of valence electrons, we must explore the outermost layer of electrons, known as the valence shell. Electrons in this shell play a pivotal role in chemical interactions, determining the element’s ability to bond with others. The number of valence electrons is directly related to the atom’s atomic number.
For elements in the first three periods of the periodic table:
- The atomic number directly corresponds to the number of valence electrons.
- For instance, sodium (Na) has an atomic number of 11, meaning it has 11 electrons in total and 1 valence electron in the outermost shell.
For elements in higher periods:
- The number of valence electrons is equal to the group number (column number) in the periodic table.
- For example, oxygen (O) is in Group 16, indicating that it has 6 valence electrons.
Electron Configuration and the Valence Shell
In the realm of chemistry, understanding the electron configuration of an atom is crucial. It provides insights into the chemical properties and behavior of an element. Every atom comprises a nucleus surrounded by electrons that occupy specific energy levels or shells. The outermost shell, known as the valence shell, plays a pivotal role in determining the number of valence electrons, which in turn governs an element’s chemical reactivity.
The electron configuration of an atom is represented by a set of numbers that indicate the number of electrons in each shell. For instance, the electron configuration of helium, an inert gas, is 1s2. This implies that helium has two electrons in its first and only shell. The number of valence electrons corresponds to the number of electrons in the outermost shell. In the case of helium, it has two valence electrons.
The position of an element in the periodic table significantly influences its electron configuration and valence electrons. Elements belonging to the same group or column have a similar number of valence electrons. For instance, the elements in Group 1 (alkali metals) all have one valence electron, while those in Group 17 (halogens) have seven valence electrons. This consistency in valence electrons leads to common chemical properties within each group.
The valence electrons are involved in chemical bonding, as they determine an element’s ability to share electrons or form ions. Elements with a high number of valence electrons tend to be more reactive, while those with a low number of valence electrons are less reactive. The understanding of electron configuration and valence electrons is fundamental to comprehending the chemical behavior of elements and their interactions with other elements.
Valence Electrons and Strontium: Unveiling the Secrets of Chemical Behavior
Let’s immerse ourselves in the fascinating world of chemistry by exploring the element strontium (Sr). This intriguing element, graced with the atomic number 38, resides in the periodic table’s Group 2, sharing a cozy spot with other alkaline earth metals.
Calculating the Number of Electrons in a Strontium Atom
Understanding the number of electrons within a strontium atom is crucial. And here’s how we crack the code: The atomic number, a unique identity for each element, gives us a direct count of the protons in its nucleus. Since atoms maintain a neutral state, the number of electrons exactly matches the number of protons. Thus, strontium atoms boast 38 electrons dancing around their nuclei.
Electron Configuration and the Valence Shell
To unveil the secret of strontium’s valence electrons, we delve into the intricate structure of its atom. Imagine a bustling city teeming with electrons, each with its designated address. This address, known as the electron configuration, describes the arrangement of electrons in energy levels surrounding the nucleus.
Understanding Electron Configuration
Strontium’s electron configuration reads like an atomic blueprint: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s². This string of numbers and letters represents the number of electrons in each energy level, starting from the innermost (1s) to the outermost (5s).
Identifying the Valence Shell
The outermost energy level, the valence shell, holds the key to understanding valence electrons. In strontium’s case, the valence shell resides at the 5s level. The electrons occupying this outermost shell are the valence electrons, the ones responsible for chemical bonding and reactivity.
Valence Electrons of Strontium
Strontium’s valence shell has a cozy population of two valence electrons. These electrons, like tiny ambassadors, venture beyond the atom’s boundaries to interact with other atoms, forging chemical bonds and shaping its unique chemical behavior.
The number of valence electrons in strontium has a profound impact on its chemical behavior. With a mere two valence electrons, strontium readily forms bonds with other elements, seeking to achieve a stable electron configuration. This tendency to donate its valence electrons makes strontium a reactive element, eager to participate in chemical reactions.
Wrap-Up
Our exploration into the world of valence electrons and strontium has shed light on the intricate relationship between atomic structure and chemical behavior. Strontium’s two valence electrons bestow upon it a unique chemical character, influencing its reactivity and shaping its role in the vast tapestry of chemistry.
Valence Electrons of Strontium: A Step-by-Step Explanation
In the realm of chemistry, valence electrons hold the key to understanding an element’s chemical behavior. These are the electrons in an atom’s outermost energy level, and they govern how the atom interacts with others.
Strontium (Sr), a member of the alkaline earth metals in Group 2 of the periodic table, boasts an atomic number of 38. This number corresponds to the number of electrons in a neutral strontium atom.
To unravel the mystery of strontium’s valence electrons, we delve into its electron configuration. This configuration depicts the arrangement of electrons in the atom’s energy levels, starting from the innermost level. Strontium’s electron configuration is:
1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>4s<sup>2</sup>3d<sup>10</sup>4p<sup>6</sup>5s<sup>2</sup>
Pay close attention to the 5s² subshell. This outermost energy level houses strontium’s valence electrons. Two valence electrons are present, and they reside in the s orbital.
The presence of two valence electrons is a defining characteristic of alkaline earth metals. This electron arrangement makes strontium highly reactive, as it eagerly donates its valence electrons to achieve a stable octet configuration.
In summary, strontium possesses two valence electrons located in its 5s orbital. These valence electrons play a crucial role in determining its chemical properties and reactivity.
Periodic Table and Alkaline Earth Metals
- Organization of elements in the periodic table according to atomic number
- Characteristics of Group 2 alkaline earth metals, including their tendency to have two valence electrons
Periodic Table and Alkaline Earth Metals
The periodic table is a systematic arrangement of chemical elements, organized according to atomic number. It provides a wealth of information about elements, including their valency and chemical reactivity.
One intriguing group on the periodic table is the Group 2 alkaline earth metals. These elements share a common feature: they tend to have two valence electrons. Valence electrons are the electrons in an atom’s outermost energy level, and they play a crucial role in determining an element’s chemical behavior.
Among the alkaline earth metals, strontium (Sr) stands out. Its atomic number of 38 reveals that it has a total of 38 electrons, with the two outermost electrons being its valence electrons. This electronic configuration gives strontium its characteristic reactivity and shapes its role in various chemical reactions.
The alkaline earth metals’ tendency to have two valence electrons is evident in their location on the periodic table. They are located in the second group, which corresponds to elements with two electrons in their outermost energy level. This shared characteristic results in similar chemical properties for all alkaline earth metals, including strontium.