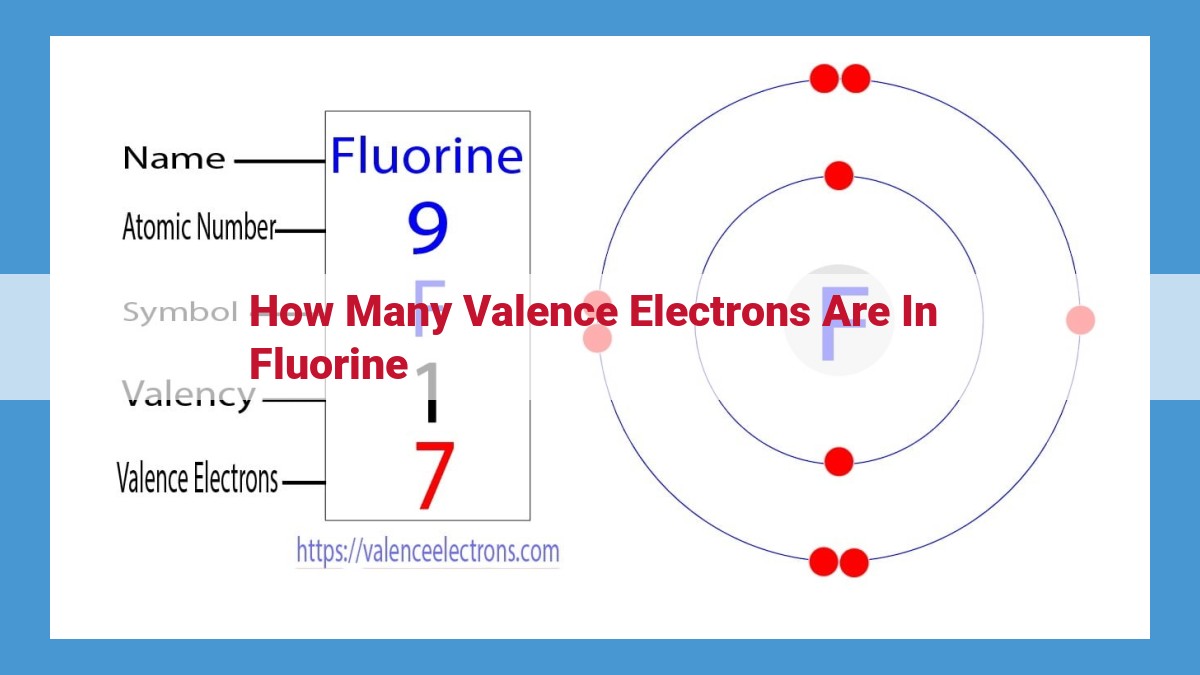
Valence electrons, crucial for chemical bonding, are the electrons in an atom’s outermost energy level. Fluorine, with an electron configuration of 1s²2s²2p⁵, has seven valence electrons, attributed to its position in Group 17 of the periodic table. These electrons play a significant role in forming strong covalent bonds with other elements, as fluorine’s high electronegativity attracts electrons toward itself, resulting in stable molecular structures. Examples include hydrogen fluoride (HF), a common acid, and calcium fluoride (CaF₂), a widely used optical material.
Valence Electrons: The Architects of Chemistry
In the vast and intricate realm of chemistry, the dance of electrons holds the key to understanding the behavior and interactions of elements. Among these electrons, a special group called valence electrons plays a pivotal role in shaping the chemical landscape. These are the electrons that occupy the outermost energy level of an atom, and they are the architects of the chemical bonds that form the building blocks of our world.
Imagine atoms as miniature planets, with their valence electrons orbiting like celestial bodies in the outermost rings. These electrons are the explorers, venturing into the vast expanse of space, seeking out other atoms to bond with. Their interactions give rise to the astonishing diversity of substances that make up our universe, from the shimmering stars above to the very ground beneath our feet.
Why Are Valence Electrons So Important?
The number of valence electrons an atom possesses profoundly influences its chemical properties. It determines the element’s ability to form bonds, the strength of those bonds, and the types of compounds it can participate in. In essence, valence electrons act as the social butterflies of the atomic world, determining which atoms will mingle and how.
Understanding the concept of valence electrons is crucial for comprehending the fundamental principles of chemistry. It provides the foundation for understanding chemical reactions, bonding, and the properties of matter. So, let’s delve deeper into this fascinating topic, starting with the enigmatic element that has only one valence electron: fluorine.
Fluorine’s Electron Configuration:
- Describe the electron configuration of fluorine, focusing on its valence electrons in the outermost energy level.
Fluorine’s Electron Configuration: A Tale of Outermost Energy
In the realm of chemistry, valence electrons play a pivotal role, like the key players in a grand symphony. They are the electrons that reside in the outermost energy level of an atom, eagerly awaiting the opportunity to engage in the dance of chemical bonding. Among the elements, fluorine stands out as a prime example of the importance of valence electrons.
Fluorine, a halogen element, proudly resides in Group 17 of the periodic table. This strategic location holds a clue to its electronic makeup. The number of the group in which an element resides corresponds directly to the number of valence electrons it possesses. In fluorine’s case, its home in Group 17 indicates that it has seven valence electrons.
These seven valence electrons are the driving force behind fluorine’s chemical behavior. They are the eager participants in the formation of chemical bonds, the molecular glue that holds atoms together. Fluorine’s high electronegativity, a measure of its attraction for electrons, further enhances its bonding prowess.
To visualize fluorine’s electron configuration, imagine an atomic nucleus surrounded by a series of concentric energy levels, like the layers of an onion. The valence electrons reside in the outermost energy level, eager to interact with their surroundings. This arrangement sets the stage for fluorine’s remarkable ability to form strong covalent bonds, sharing electrons with other atoms to create stable molecules.
Unveiling Fluorine’s Versatile Nature: A Journey Through Its Valence Electrons
In the fascinating world of chemistry, valence electrons hold the key to understanding an element’s chemical behavior. These electrons, residing in the outermost energy level, dictate the element’s bonding capabilities and reactivity. Let’s delve into the realm of fluorine, a highly electronegative element with a unique set of valence electrons that determine its chemical personality.
Fluorine’s Electron Configuration: A Blueprint of Valence Electrons
Fluorine, an element found in Group 17 of the Periodic Table, boasts an atomic number of 9. This number reveals its electron configuration:
1s² 2s² 2p⁵
Here, the superscripts denote the number of electrons in each energy level, with the 2p⁵ indicating that fluorine has five valence electrons in its outermost energy level.
Periodic Table Magic: Predicting Valence Electrons
The Periodic Table serves as a valuable tool for predicting an element’s valence electrons. Elements belonging to the same group (vertical column) share a similar number of valence electrons. Fluorine, being in Group 17, the Halogens, has seven valence electrons. This crucial piece of information provides a glimpse into fluorine’s chemical versatility.
The Role of Valence Electrons in Chemical Bonding: Unveiling the Secrets of Connectivity
Valence electrons, like the social butterflies of chemistry, play a pivotal role in forging bonds between atoms. These electrons reside in the outermost energy level of an atom, eager to engage in chemical interactions. They determine an element’s electronegativity, a measure of its ability to attract those precious electrons.
Fluorine, the vibrant element that makes up the bulk of Teflon’s non-stick magic, stands as a prime example of valence electrons’ bonding prowess. Fluorine possesses seven valence electrons, relentlessly seeking partners to form strong covalent bonds. These bonds arise when atoms share their valence electrons, creating a molecular dance that holds the structure together.
As a highly electronegative element, fluorine has a strong desire to dominate its electrons. This tendency leads to an overwhelming pull towards the bonding electrons, giving fluorine a firm grip on its chemical bonds. This characteristic makes fluorine an excellent partner for forming stable, durable materials like hydrofluoric acid and fluorinated polymers.
In the grand tapestry of chemistry, valence electrons are the threads that weave the intricate fabric of molecular interactions. By understanding the role of these electrons in bonding, we gain a deeper appreciation for the diverse and fascinating world of chemical substances that surround us.
Fluorine: The Element with the Strongest Bonds
Fluorine (F) is a highly reactive element due to its unique electron configuration, which gives it an unusual number of valence electrons. Valence electrons are the outermost electrons in an atom’s energy level, and they play a crucial role in determining an element’s chemical properties. Fluorine’s seven valence electrons make it highly electronegative, meaning it has a strong tendency to attract electrons from other atoms. This property allows fluorine to form strong covalent bonds with other elements.
Fluorine’s Electron Configuration and Valence Electrons
Fluorine has nine electrons, with two in the first energy level, two in the second, and five in the third. The five electrons in the third energy level are its valence electrons. Elements in the same periodic table group have the same number of valence electrons, and fluorine belongs to Group 17 (the halogens), which means it has seven valence electrons.
Covalent Bonding in Fluorine
Fluorine’s high electronegativity means it readily forms covalent bonds, in which atoms share electrons to achieve a stable electron configuration. In covalent bonding, each atom contributes one or more valence electrons to form a shared electron pair. This shared pair of electrons is attracted to the nuclei of both atoms, forming a covalent bond.
Specific Examples of Fluorine Bonding
-
Hydrogen fluoride (HF): Fluorine’s high electronegativity allows it to form a strong covalent bond with hydrogen in hydrogen fluoride. HF is a highly corrosive acid used in the production of semiconductors and plastics.
-
Sodium fluoride (NaF): Fluorine bonds with sodium, a highly electropositive element, to form sodium fluoride. NaF is used in toothpaste and mouthwash to strengthen teeth and prevent cavities.
-
Calcium fluoride (CaF2): Fluorine’s high electronegativity enables it to form a covalent bond with calcium in calcium fluoride. CaF2 is used as a flux in metallurgy and as an optical material.
Fluorine’s seven valence electrons and high electronegativity make it a highly reactive element that readily forms strong covalent bonds with other elements. These bonds are responsible for fluorine’s unique chemical properties, which are exploited in a variety of industrial and household applications. By understanding the role of valence electrons in fluorine’s bonding, we can gain insights into its chemical behavior and practical applications.