How Many Valence Electrons Does Li Have?
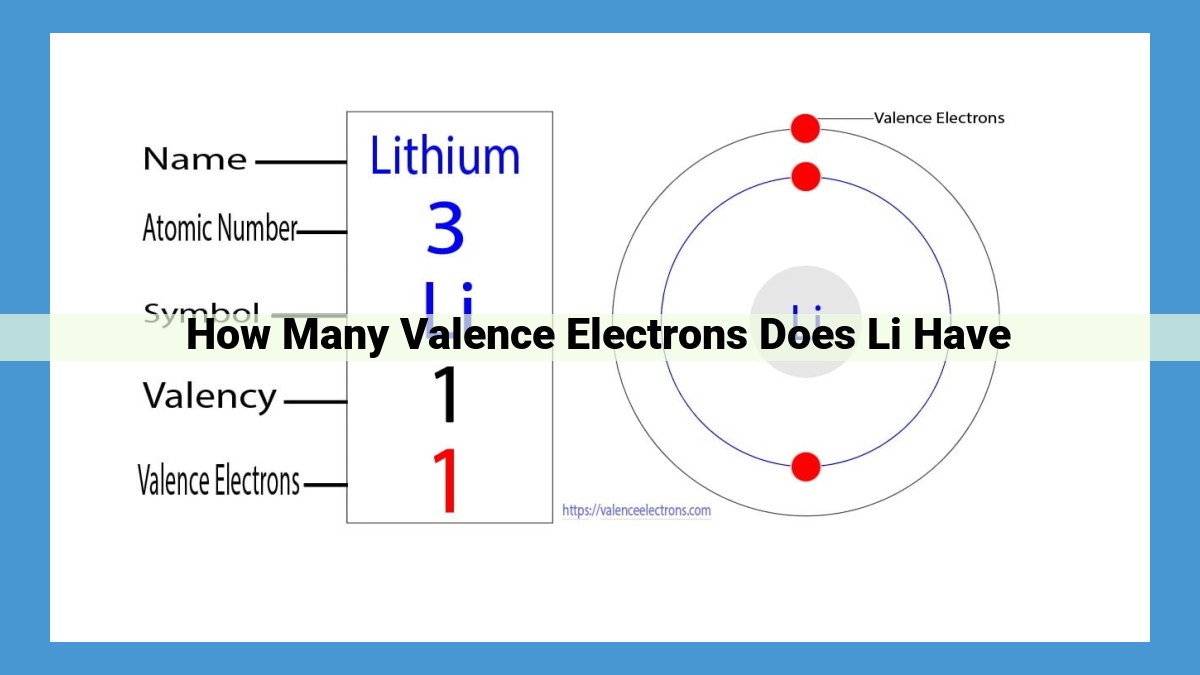
Lithium (Li), the lightest element in Group 1, possesses a unique electron configuration that determines its chemical properties. With an atomic number of 3, Li has three electrons, one of which is located in the outermost energy level. Valence electrons, those in the outermost level, play a crucial role in chemical bonding. Li’s single valence electron allows it to interact with other atoms, forming bonds that stabilize its configuration and contribute to its chemical reactivity.
How Many Valence Electrons Does Lithium Have?
Imagine you’re in a bustling city, where each person represents an electron. These electrons are like tiny energy packets that orbit a central nucleus, which is like the city’s core. The electrons occupy different energy levels, or “floors” in this metaphorical city. The outermost floor, akin to the penthouse suite, is where we find valence electrons.
These valence electrons play a crucial role in determining how atoms interact with one another, influencing their chemical bonding abilities. They’re like the social butterflies of the atomic world, eager to connect with other atoms.
One of the lightest and most sociable elements is lithium (Li), the first resident of Group 1 on the periodic table. It has a total of three electrons, residing in two energy levels. The penthouse suite, or the outermost energy level, accommodates a single valence electron. This one lonely electron is like the city’s resident extrovert, always ready to mingle and form bonds.
Lithium’s atomic number, which represents the number of electrons, is 3. Its electron configuration, or the arrangement of electrons in energy levels, is 1s²2s¹. The “1s” and “2s” denote the energy level, while the superscripts indicate the number of electrons in each level.
The lone valence electron in lithium’s 2s orbital is what makes this element so reactive. It’s like an eager participant in a game of musical chairs, always seeking to complete its noble gas configuration, which consists of a full set of eight valence electrons. In this case, lithium would mimic the stable electron configuration of its noble gas neighbor, helium.
To achieve this stable configuration, lithium is willing to share its valence electron with other atoms. This chemical bonding tendency is what makes lithium an essential component in many batteries, as its ions can move freely, facilitating the flow of electricity.
In conclusion, lithium has one valence electron. This single electron plays a pivotal role in lithium’s chemical behavior, influencing its reactivity and ability to form bonds with other atoms. Valence electrons are fundamental to understanding the interactions between atoms and the fascinating world of chemistry.
Lithium: Unveiling the Secrets of Valence Electrons
In the realm of chemistry, valence electrons play a pivotal role in shaping the behavior and bonding capabilities of elements. These electrons reside in the outermost energy level of an atom, eagerly seeking partners to forge chemical connections.
Lithium (Li), the lightest element in Group 1, holds a special place in this atomic dance. With a mere three electrons, this element stands out as a chemical chameleon. Its single valence electron grants it the power to bond with various elements, creating a diverse range of compounds.
Atomically speaking, lithium boasts three protons in its nucleus, lending it an atomic number of 3. This atomic number also determines the total number of electrons in a neutral lithium atom: three. Two of these electrons occupy the 1s orbital, forming a stable inner core. The remaining electron ventures into the 2s orbital, becoming the solitary valence electron.
This lone valence electron yearns for companionship, eagerly seeking chemical partners to complete its outermost energy level. In the quest for stability, lithium readily interacts with other elements, forming ionic bonds by transferring its valence electron to achieve a noble gas configuration—the coveted state of eight valence electrons.
For instance, when lithium encounters chlorine, it happily donates its valence electron to chlorine’s seven valence electrons. This transfer results in the formation of lithium chloride (LiCl), with both lithium and chlorine attaining the coveted noble gas configuration of helium and neon, respectively.
Electron Configuration: Unraveling the Mystery of Lithium’s Valence Electrons
What’s an Electron Configuration, Anyway?
Imagine an atom as a tiny solar system, with a nucleus as its sun and electrons as planets orbiting around it. Electron configuration is the roadmap that tells us how these electrons are arranged in different energy levels, or “orbits.” It’s like a cosmic dance, with each electron seeking its preferred energy level.
Lithium: The Lone Ranger Electron
Let’s zoom in on lithium (Li), the lightest element in the periodic table. With just three electrons in its arsenal, it’s the cosmic equivalent of a minimalist. Lithium’s electron configuration is as simple as it gets: 1s²2s¹.
The first two electrons (1s²) reside in the innermost energy level, snuggled up like warm and fuzzy kittens. But the third electron (2s¹) stands out like a solitary star, occupying a higher energy level. This lonely electron is the key to understanding lithium’s chemistry.
Significance of Valence Electrons
Valence electrons are the electrons that reside in the outermost energy level, like a ring of stars encircling an atom. They play a crucial role in chemical bonding, the dance that atoms perform to form molecules. Like a shy high schooler waiting to mingle, lithium’s one valence electron yearns to interact with other atoms.
Noble Gas Configuration and the Quest for Stability
Atoms strive for stability, and they can achieve this by mirroring the electron configuration of noble gases, elements like helium that are known for their chemical inertness. For lithium, the noble gas configuration of helium (1s²) is just one electron away.
Lewis Structure: Visualizing Lithium’s Lone Electron
A Lewis structure is a handy tool that shows the arrangement of valence electrons as dots around an atomic symbol. Lithium’s Lewis structure is simply a single dot, representing its lonely valence electron. This dot symbolizing the electron’s eagerness to bond with others.
Chemical Bonding: Lithium’s Dance Partners
In the world of chemistry, atoms join hands to form molecules through the sharing or transfer of valence electrons. Lithium’s single valence electron makes it a willing partner in a variety of chemical bonds, enabling it to interact with various elements.
Valence Electrons
- Identification as electrons in the outermost energy level
- Lithium’s one valence electron
Valence Electrons: Unveiling Lithium’s Chemical Identity
In the realm of chemistry, valence electrons play a pivotal role, determining an element’s chemical personality and its ability to form bonds with other elements. Let’s embark on a journey to understand valence electrons, using the element lithium as our guide.
What are Valence Electrons?
Imagine an atom as a miniature solar system, with the nucleus as the sun and electrons orbiting it like planets. The outermost energy level of an atom, known as the valence shell, holds the key to understanding valence electrons. These are the electrons that participate in chemical bonding, giving elements their unique chemical characteristics.
Lithium’s Valence Electron
Lithium (Li) is the lightest element in Group 1 of the periodic table, possessing an atomic number of 3. This means that Li has three electrons, with two in the first energy level and one in the second energy level. Hence, Li has one valence electron.
Electron Configuration and the Path to Stability
The arrangement of electrons in an atom’s energy levels is called its electron configuration. Li‘s electron configuration is 1s²2s¹. The presence of a single valence electron makes Li highly reactive, as it seeks to achieve a stable electron configuration similar to that of helium (He). He, with two electrons in its first energy level, represents a stable configuration for the first row elements.
Lewis Structure: A Visual Representation of Valence Electrons
To visualize the distribution of valence electrons, chemists use Lewis structures. These diagrams show the atoms involved in a bond and their valence electrons as dots. For Li, the Lewis structure is simply a dot (Li̇), representing its single valence electron.
Importance of Valence Electrons in Chemical Bonding
Valence electrons play a crucial role in chemical bonding. Atoms interact to achieve a stable electron configuration, usually by gaining or sharing valence electrons. Li‘s single valence electron makes it a good candidate for bonding with other elements to attain a stable octet configuration (eight valence electrons).
The concept of valence electrons is fundamental to understanding the chemical behavior of elements. Lithium‘s one valence electron shapes its reactivity and bonding properties, making it an essential player in various chemical processes. By unraveling the mysteries of valence electrons, we unravel the secrets of chemical bonding and the fascinating world of chemistry.
Noble Gas Configuration: Lithium’s Affinity to Helium’s Stability
As we delve into the world of chemistry, understanding the concept of valence electrons becomes crucial. These electrons, residing in the outermost energy level of an atom, play a pivotal role in determining an element’s chemical behavior. In this exploration, we’ll explore Lithium (Li), the lightest member of Group 1, and unravel the significance of its single valence electron.
Electron Configuration and Valence Electrons
Every element possesses a unique electron configuration, representing the distribution of its electrons within different energy levels. Lithium’s electron configuration is 1s²2s¹. This means that it has two electrons in the first energy level (1s) and one electron in the second energy level (2s). This lone electron in the 2s orbital is Li’s valence electron.
Proximity to Helium’s Noble Gas Configuration
In the world of chemistry, achieving a noble gas configuration is akin to reaching a state of stability and chemical inertness. Noble gases, with their full outer electron shells, exhibit minimal reactivity. Helium, with its two valence electrons, possesses a particularly stable configuration.
Lithium’s Noble Gas Affinity
Lithium, with its single valence electron, is just one electron shy of reaching helium’s stable configuration. This proximity to a noble gas configuration significantly influences Li’s chemical behavior. In its quest for stability, Lithium tends to lose its valence electron, forming Li⁺ cations.
Chemical Bonding and the Valence Electron
Valence electrons are the key players in chemical bonding, the process by which atoms combine to form molecules or compounds. Lithium’s single valence electron allows it to participate in various bonding interactions, including ionic bonding and covalent bonding.
Lithium’s single valence electron not only determines its chemical reactivity but also highlights the importance of valence electrons in shaping the chemical landscape. Understanding the concept of valence electrons and their role in electron configuration and noble gas configurations is essential for comprehending the fundamental principles of chemistry and unraveling the intricate dance of elements in the world around us.
Lithium’s Lone Electron: A Gateway to Chemical Bonding
In the realm of chemistry, understanding electron configurations and valence electrons is crucial. Valence electrons hold the key to unlocking the secrets of chemical bonding and determining the properties of elements. And there’s no better place to start our exploration than with the lightest element of all: lithium (Li).
Lithium: The Element with a Single Mission
Lithium is a fascinating element that belongs to Group 1 of the periodic table. Its atomic number of 3 reveals that it possesses three electrons, two of which reside in the innermost energy level and one ventures into the outermost level. This lone electron in the outermost shell is the defining characteristic of lithium, giving it its unique chemical properties.
Electron Configuration: A Map to Lithium’s Valence
The electron configuration of lithium, written as 1s²2s¹, provides a blueprint of its electron distribution. The “1s²” portion indicates two electrons comfortably nestled in the first energy level, while the “2s¹” signifies the lone valence electron in the second energy level.
Valence Electrons: The Gatekeepers of Bonding
Valence electrons, as their name suggests, are those electrons that determine an element’s chemical bonding behavior. They reside in the outermost energy level and are the participants in the bonding process. Lithium’s single valence electron is what makes it a highly reactive element, eager to form bonds with other atoms to achieve a stable configuration.
Noble Gas Ambition: Lithium’s Path to Stability
Elements strive to attain the noble gas configuration, a stable arrangement of electrons that resembles the electron configuration of the noble gases (e.g., helium, neon). Lithium, with its one valence electron, is just a step away from achieving this stability. By gaining one electron, it can mirror helium’s stable “1s²” configuration.
Lewis Structure: A Visual Representation of Lithium’s Valence Electron
Lewis structures are pictorial representations that depict the valence electrons of atoms within a molecule. For lithium, the Lewis structure simply features one dot, representing its lone valence electron. This single dot symbolizes lithium’s readiness to participate in chemical bonding and its pursuit of a stable electron configuration.
Chemical Bonding: Lithium’s Dance with Other Atoms
Lithium’s valence electron is the driving force behind its chemical bonding behavior. It can engage in various types of bonding, such as ionic bonding and covalent bonding, to achieve a stable configuration and form compounds. By sharing or transferring its valence electron, lithium can forge bonds with other atoms and create molecules with unique properties.
Lithium’s single valence electron is the defining characteristic that governs its chemical behavior. This lone electron enables lithium to form bonds with other atoms, creating compounds with diverse properties. Understanding the role of valence electrons is fundamental in chemistry, as they dictate the bonding interactions between elements and ultimately shape the world of chemical reactions.
Chemical Bonding
Valence electrons play a pivotal role in the dance of chemical bonding. They are the social butterflies of the atomic world, determining how elements interact with each other to form the myriad compounds that make up our universe.
In the case of lithium, its single valence electron is the key to its chemical personality. This lone electron, yearning for companionship, eagerly seeks to share its presence with other atoms, forming bonds that create new substances with unique properties.
Lithium’s single valence electron allows it to participate in ionic bonding, where it donates its electron to a more electronegative atom, such as fluorine. This creates two oppositely charged ions that are attracted to each other, forming a strong ionic bond.
Lithium’s single valence electron also enables it to engage in covalent bonding, where it shares its electron with another atom to form a covalent bond. In this type of bonding, the shared electron pair is attracted to both atoms, creating a stable molecular bond.