To draw oxygen, understand its significance and depict it as a light blue or white circle. Delve into its atomic structure, illustrating 8 protons, neutrons, and electrons as circles within the atom. Then, represent the oxygen molecule as two bonded circles. To capture its gaseous state, use a cloud of dots around the molecule. Employ simple shapes, accurate sizing, and refer to scientific resources for guidance. These drawings enhance understanding and serve educational, scientific, and artistic purposes.
Understanding Oxygen’s Role
Oxygen, an indispensable element for life on Earth, plays a pivotal role in sustaining the vibrant tapestry of life on our planet. It fuels the metabolic processes of all aerobic organisms, providing the energy that drives the intricate machinery of life. Beyond its biological significance, understanding the structure and properties of oxygen is crucial for creating accurate drawings.
By delving into the atomic and molecular makeup of oxygen, we can unravel the complexities of its structure and translate them into visual representations that capture its unique characteristics. Accurate drawings not only aid in scientific understanding but also serve as valuable tools for educational purposes and artistic endeavors.
Basic Characteristics of Oxygen: Unveiling the Essence of Life
Oxygen, the lifeblood of our planet, is an essential element that permeates every aspect of our existence. Understanding its fundamental characteristics is crucial for comprehending its profound impact on our world.
Invisible Yet Omnipresent
Oxygen exists as a colorless, odorless, and tasteless gas, making it invisible to our senses. Despite its elusiveness, it is the most abundant element in Earth’s atmosphere, constituting approximately 21%. Its prevalence makes it an omnipresent force in our environment.
From Light Blue to Pristine White
In scientific depictions, oxygen is often represented in light blue or white. This convention arises from the way oxygen absorbs and reflects light. When white light passes through oxygen, certain wavelengths are absorbed, leaving behind a faint bluish hue. In contrast, in pure form, oxygen appears colorless, giving it a pristine white appearance.
The Circle of Life
Oxygen’s basic shape is typically depicted as a circle or a sphere. This geometric simplicity reflects the fundamental structure of oxygen atoms. Each atom consists of a dense nucleus surrounded by a cloud of electrons. In simplified drawings, the nucleus is represented by a small circle or dot, while the electrons are depicted as a series of circles or loops around it.
By understanding the basic characteristics of oxygen, we gain a deeper appreciation for its unseen yet omnipresent presence and its profound role in sustaining life on our planet.
Delving into Oxygen’s Atomic Structure
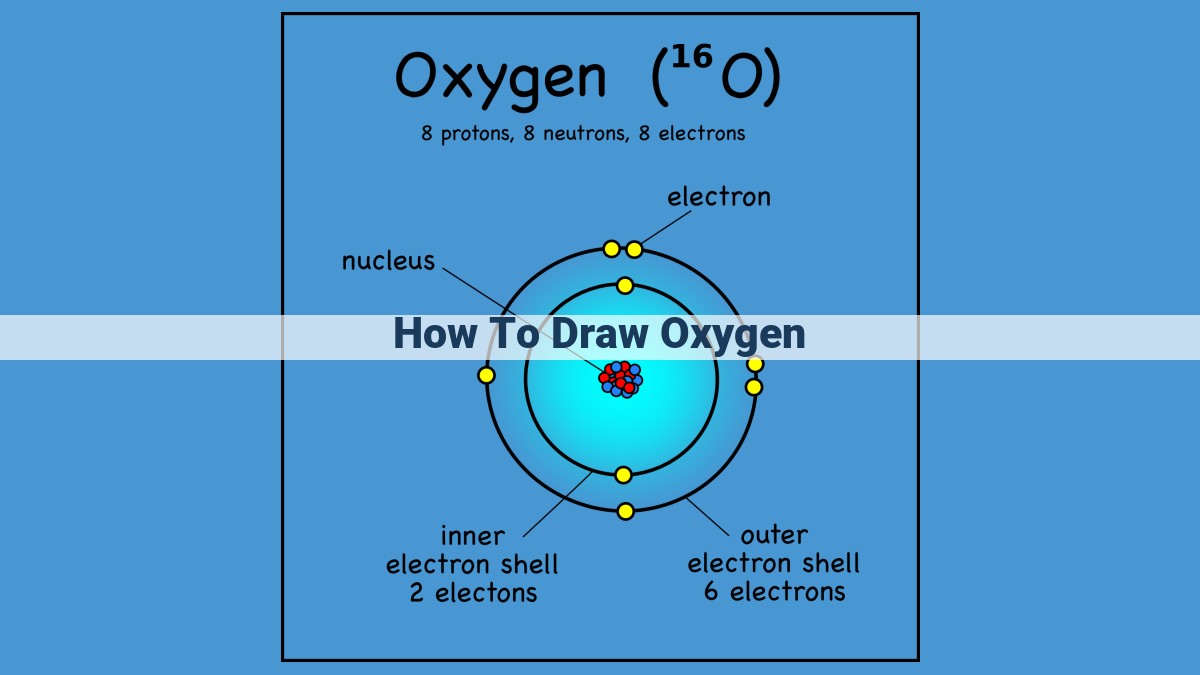
At the very core of oxygen lies its atomic structure, a symphony of subatomic particles that determine its unique properties. Understanding this atomic makeup is crucial for accurate depictions in drawings.
The oxygen atom is a bustling hub of 16 fundamental entities: 8 protons, which reside in the nucleus and harbor a positive charge, and 8 neutrons, their neutral counterparts, also nestled in the nucleus. These particles form the atom’s weighty core.
Encircling this nuclear heart are 8 electrons, graceful particles that dance in continuous motion around the nucleus. Electrons, with their negative charge, balance the positive charge of the protons, creating a harmonious symphony within the atom.
To visualize this atomic structure, imagine a serene lake, its shimmering surface representing the outermost electron shell. Within this lake, eight vibrant lily pads emerge, each symbolizing an electron. The tranquil waters beneath hold the atom’s nucleus, where eight protons and eight neutrons reside like steadfast rocks, anchored in the center of the atom.
Representing this atomic structure in drawings is straightforward:
- Draw a circle to represent the nucleus, with the protons and neutrons indicated as dots within the circle.
- Draw eight smaller circles around the nucleus, representing the electron shell.
- You can also depict the electrons as dots or lines orbiting the nucleus.
With this atomic blueprint in mind, we can delve into the molecular structure of oxygen, exploring the dance between individual atoms.
Examining Oxygen’s Molecular Structure
Delving further into the world of oxygen, we encounter its molecular structure, which forms the foundation of how oxygen exists in nature. An oxygen molecule is a fascinating entity, consisting of two oxygen atoms covalently bonded together. This bond is formed when the atoms share electrons, creating a stable and robust entity.
To draw an oxygen molecule, follow these simple steps:
- Draw two circles. These circles represent the two oxygen atoms.
- Connect the circles with a line. This line represents the covalent bond between the atoms.
Remember, the relative sizes of the circles should be accurate, with the circles representing the oxygen atoms being larger than the line representing the bond. This helps convey the proportional differences in size between the atoms and the bond.
Tips and Tricks
Here are a few tips for effective drawings of oxygen molecules:
- Use simple shapes and lines for clarity.
- Maintain accurate relative sizes of atoms and molecules.
- Refer to scientific resources for guidance.
Example:
Consider the following diagram of an oxygen molecule:
O-O
The circles represent the oxygen atoms, and the line connecting them represents the covalent bond. This drawing effectively conveys the structure and characteristics of an oxygen molecule.
Drawing oxygen molecules is essential for understanding their structure and properties. These drawings find applications in education, science, and art, helping us visualize and comprehend the fundamental building blocks of life on Earth.
Depicting Oxygen’s Elusive Gaseous State
In the realm of scientific illustration, accurately portraying oxygen’s enigmatic gaseous state poses a unique challenge. Unlike its tangible counterparts, oxygen exists as an invisible entity, its presence detectable only through its interactions with other substances. To capture this elusive quality, artists and scientists alike have devised ingenious techniques that convey its gaseous nature with visual clarity.
One of the most effective methods involves surrounding the oxygen molecule with a cloud of tiny dots. These dots, representing individual oxygen atoms, are depicted in random motion, mimicking the ceaseless movement of gas particles. By using this technique, illustrators create a sense of the oxygen’s fluidity and its tendency to expand and fill any available space.
The key to accurately depicting oxygen’s gaseous state lies in maintaining consistency in the size and arrangement of the dots. The dots should be small enough to suggest a multitude of particles, yet large enough to be clearly visible. The spacing between the dots should also be consistent, conveying the random motion of the gas particles without creating an overly crowded or chaotic appearance.
By employing these simple techniques, artists can effectively capture the elusive gaseous nature of oxygen, bringing it to life in vivid detail. Whether for educational purposes, scientific illustrations, or artistic expressions, these depictions serve as a testament to the power of visual representation in conveying complex scientific concepts.
Tips and Examples for Effective Oxygen Drawings
Understanding the intricacies of oxygen’s structure and properties is fundamental for creating accurate drawings. Follow these tips to enhance your artistic renditions:
Use Simple Shapes and Lines
When drawing oxygen, opt for simple geometric shapes like circles and spheres. These basic forms effectively represent the atom’s spherical shape and the molecule’s elongated structure. Avoid unnecessary details to maintain clarity.
Maintain Relative Size
Accurately depict the relative sizes of subatomic particles and molecules. The oxygen atom should be significantly smaller than the oxygen molecule, which consists of two bonded oxygen atoms.
Refer to Scientific Resources
Consult scientific diagrams and reference materials to guide your drawings. These resources provide accurate representations of oxygen’s structure at different levels, ensuring your drawings align with scientific knowledge.
Examples
Here’s an example of an effective oxygen drawing:
Oxygen Atom: Draw a small circle representing the nucleus, containing the protons and neutrons. Surround the nucleus with a larger circle representing the electron cloud.
Oxygen Molecule: Draw two overlapping circles connected by a single line. These circles represent the two oxygen atoms bonded together.
Tips for Gas State
In its gaseous state, oxygen exists as a collection of unattached molecules randomly moving. Represent this by drawing a cloud of dots around the oxygen molecule. The dots should be evenly distributed to convey the chaotic motion of the gas particles.