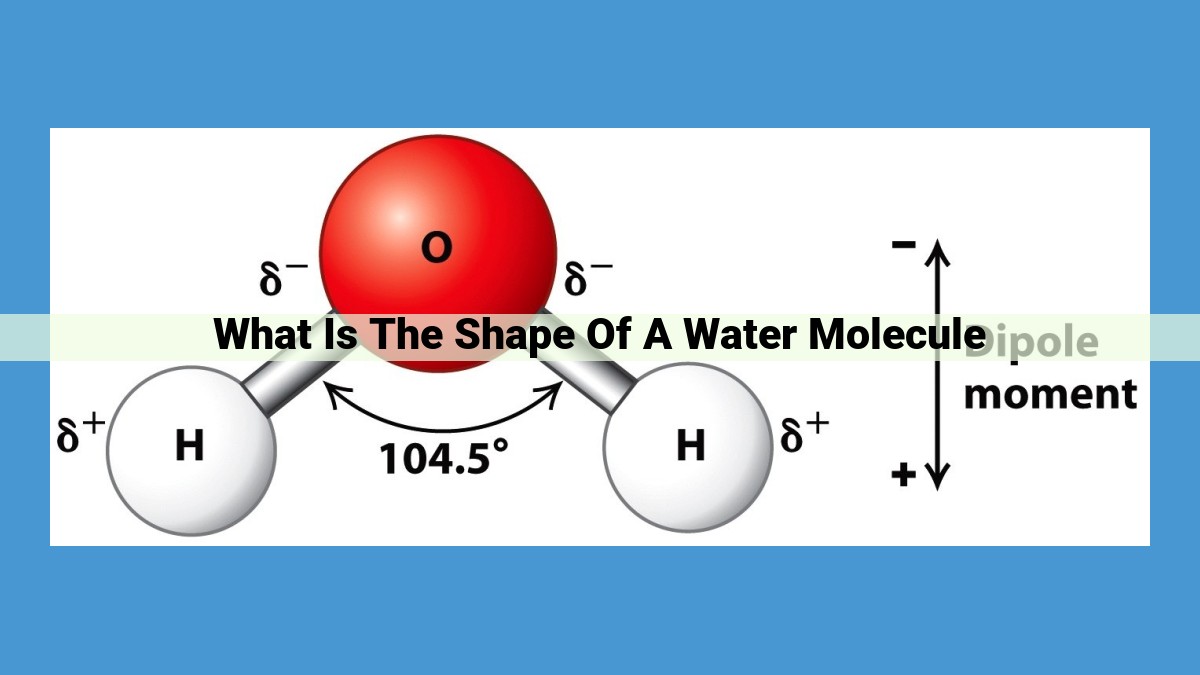
A water molecule has a bent shape, resembling a “V.” This unique V-shape arises from the repulsion between the electron pairs surrounding the oxygen atom, forcing the two hydrogen atoms to form a 104.5-degree angle with each other. This bent shape contributes to water’s polarity, as the covalent bonds between oxygen and hydrogen create a slight positive charge on the hydrogen atoms and a slight negative charge on the oxygen atom. This molecular polarity, along with water’s ability to form hydrogen bonds, gives rise to many of its unique physical and chemical properties.
The Intriguing World of Molecular Shapes: A Journey into the Realm of Atomic Arrangements
In the captivating realm of chemistry, the arrangement of atoms within molecules plays a crucial role in determining their properties and behavior. Molecular shape refers to the three-dimensional geometry of these atomic arrangements. Understanding molecular shape is paramount in unraveling the mysteries of chemical reactions, material properties, and biological processes.
One of the most fundamental molecular shapes is the tetrahedral shape. This iconic geometry arises when an atom is surrounded by four other atoms, forming four equivalent bonds that point towards the corners of a tetrahedron. A classic example of a tetrahedral molecule is methane (CH4) where a carbon atom sits at the center with four hydrogen atoms equidistant from each other.
Another common molecular shape is the bent shape. This configuration occurs when an atom has three electron pairs arranged around it. The electron pairs repel each other, causing the molecule to adopt a V-shaped geometry. Water is a prime example of a bent-shaped molecule, with two hydrogen atoms and two lone pairs of electrons surrounding the central oxygen atom.
Water Molecule’s Bent Shape
- Reasons behind the V-shaped configuration of water molecules
- Role of electron-pair repulsion in determining the shape
Water’s Unique V-Shaped Configuration: A Tale of Electron Dance
Imagine water, the elixir of life, a substance so familiar yet so captivating. Its molecular shape holds the key to its remarkable properties that make life on Earth possible.
Every water molecule is a symphony of electron pairs, tiny subatomic dancers that orchestrate the molecule’s geometry. Two of these electron pairs form strong bonds with hydrogen atoms, creating a V-shaped configuration. The third electron pair, however, is a loner, residing in a non-bonding orbital.
This non-bonding electron pair is like an unwelcome guest at a party. It exerts a repulsive force on the bonding electron pairs, pushing them as far apart as they can go. The result is a molecular shape that resembles a bent V, with the hydrogen atoms forming the legs and the non-bonding electron pair occupying the vertex.
This V-shaped geometry is crucial for water’s unique properties. The unshared electron pair creates an uneven distribution of electrons, resulting in a polar molecule. This polarity allows water molecules to interact with each other through hydrogen bonding, a weak but highly significant intermolecular force.
Molecular Polarity and Dipole Moments: Uncovering the Hidden Forces that Shape Matter
In the realm of chemistry, the shape of a molecule plays a pivotal role in determining its properties. Among the many factors that influence molecular shape, one stands out: molecular polarity.
What is Molecular Polarity?
Molecular polarity refers to the uneven distribution of electrons within a molecule, creating a separation of positive and negative charges. This asymmetry results in a polarity axis, much like the north and south poles of a magnet. When molecules possess such an asymmetry, they are considered polar molecules.
Dipole Moment: Quantifying Polarity
To measure the degree of polarity in a molecule, scientists use a quantity called dipole moment. The dipole moment is a vector that points in the direction of the positive charge and has a magnitude equal to the product of the magnitude of the partial charges and the distance between them. The greater the dipole moment, the more polar the molecule.
The dipole moment provides a numerical value that quantifies the polarity of a molecule. It is a crucial parameter in understanding various physical and chemical properties, such as solubility, intermolecular interactions, and molecular interactions.
Hydrogen Bonding in Water: The Key to Water’s Exceptional Properties
Water, the elixir of life, plays a vital role in sustaining life on Earth. Its unique properties, such as high boiling point, cohesion, adhesion, and solvent capabilities, stem from a remarkable intermolecular force: hydrogen bonding.
Hydrogen bonding arises when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, and is attracted to another electronegative atom nearby. This weak electrostatic attraction creates a temporary dipole-dipole interaction between the molecules involved.
In water, hydrogen bonding occurs between the partially positive hydrogen atoms of one water molecule and the partially negative oxygen atoms of another. This network of hydrogen bonds forms a three-dimensional structure that endows water with its exceptional properties.
The high boiling point of water, for example, is a result of the strong hydrogen bonds that hold water molecules together. Breaking these bonds requires a significant amount of energy, resulting in a higher boiling point compared to other liquids with similar molecular weights.
Cohesion, the attraction between water molecules, also arises from hydrogen bonding. This cohesion enables water to form droplets and cling to surfaces, allowing plants to transport water upwards through their stems.
Adhesion, the attraction between water and other substances, is another consequence of hydrogen bonding. This property enables water to wet surfaces, making it an essential component in many biological processes.
Finally, hydrogen bonding contributes to water’s exceptional solvent capabilities. The partially positive hydrogen atoms act as electron-pair acceptors, forming hydrogen bonds with polar molecules and ions. This ability to dissolve a wide range of substances makes water the universal solvent in biological systems and many industrial applications.
In summary, hydrogen bonding is a weak intermolecular force that plays a crucial role in shaping the unique properties of water. It is responsible for its high boiling point, cohesion, adhesion, and solvent capabilities, making water an indispensable substance for life on Earth.