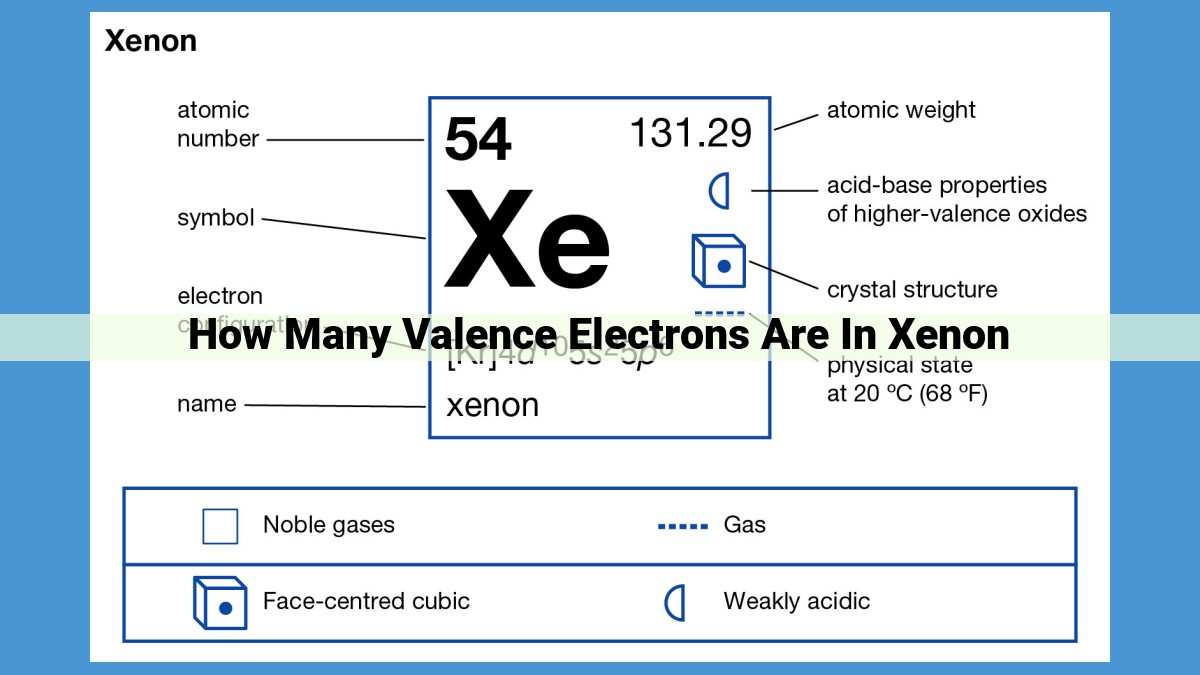
Xenon, a noble gas in Group 18 of the periodic table, exhibits unique properties due to its stable electron configuration. Noble gases, characterized by their inertness and colorless, odorless nature, possess a full outermost electron shell, giving them a filled valence electron count. Xenon, specifically, has eight valence electrons, contributing to its stability and inertness. This electron configuration aligns with the octet rule, which states that atoms tend to gain or lose electrons to achieve eight valence electrons in their outermost shell. Xenon’s electron configuration, with eight valence electrons, satisfies this rule, giving it a stable and non-reactive nature.
Valence Electrons: A Foundation
In the realm of chemistry, valence electrons play a fundamental role in shaping the interactions between atoms and molecules. These electrons reside in the outermost energy level of an atom and determine its chemical behavior.
Imagine atoms as tiny solar systems, with the nucleus at the center and the electrons orbiting around it. Valence electrons are the outermost electrons in these “orbits.” They are the most loosely held electrons and, therefore, the most likely to participate in chemical bonding.
Chemical bonding occurs when atoms share or exchange valence electrons to achieve a stable electronic configuration. For instance, when two hydrogen atoms come together to form a hydrogen molecule, they share their single valence electron, creating a covalent bond.
Understanding valence electrons is crucial for comprehending the vast array of chemical reactions that occur in the world around us.
Noble Gases: The Inert Ensemble
In the enigmatic tapestry of elements, there exists a group of gases that stand apart, cloaked in an aura of aloofness: The noble gases. These ethereal substances are the epitome of disinterest, rarely deigning to engage with their chemical brethren. Their aloofness stems from their electronic configuration, a peculiar arrangement that renders them content in their solitude.
The noble gases, nestled in Group 18 of the periodic table, are a sextet of elements: helium, neon, argon, krypton, xenon, and the enigmatic radon. Their most distinguishing characteristic is their inertness, a testament to their reluctance to partake in chemical reactions. This inertness arises from their electronic structure, which boasts a full complement of valence electrons—the outermost electrons that govern chemical bonding. Unlike their more reactive counterparts, the noble gases have no burning desire to acquire or donate electrons, hence their unwillingness to form chemical alliances.
Furthermore, noble gases are colorless, odorless, and tasteless, belying their presence in our surroundings. They are the epitome of elusive, existing as invisible entities that permeate our atmosphere, yet remain unnoticed by our senses.
Xenon: A Noble Gas with Unique Properties
Nestled within the enigmatic realm of the noble gases, Xenon stands out as a captivating element. Poised in Group 18 of the periodic table, Xenon embodies the epitome of inertness. Its colorless, odorless, and tasteless nature has earned it the moniker “the perfect gas.”
This enigmatic gas finds a myriad of applications in modern society. In the realm of medicine, Xenon serves as an anesthetic, providing pain relief during surgeries and other procedures. Its high solubility in blood enables it to diffuse rapidly throughout the body, offering effective analgesia with minimal side effects.
Moreover, Xenon’s unique properties make it an ideal choice for various industrial processes. Its ability to form stable excimers has led to its use in high-power lasers, which find applications in cutting, welding, and medical imaging. Additionally, Xenon’s high atomic number makes it an excellent radiation shield, employed in protective gear and medical equipment.
As we delve deeper into the captivating world of Xenon, its valence electrons hold the key to unlocking its remarkable stability. With a total of eight valence electrons, Xenon adheres meticulously to the octet rule, striving to achieve the stable electronic configuration of a noble gas. This configuration grants Xenon an unparalleled level of inertness, rendering it exceptionally resistant to chemical reactions.
In conclusion, Xenon stands as a testament to the remarkable diversity of the noble gases. Its unique properties and wide-ranging applications have made it an indispensable element in various scientific, medical, and industrial fields. As we continue to unravel the secrets of this enigmatic gas, its potential for future breakthroughs remains vast, promising to further enhance our understanding of the chemical world.
Exploring the Role of Valence Electrons in Xenon: Unraveling the Octet Rule
In the captivating world of chemistry, valence electrons play a pivotal role in shaping the behavior and properties of elements. The octet rule, a fundamental concept in chemistry, states that atoms strive to achieve a stable electron configuration by having eight valence electrons in their outermost shell. Xenon, a noble gas, offers a fascinating case study to explore the implications of the octet rule.
The Inert Nature of Noble Gases
Noble gases, including xenon, are renowned for their inertness. They rarely participate in chemical reactions due to their complete valence electron shells. Xenon, with eight valence electrons, epitomizes this inert nature. Its outermost electron shell is filled to capacity, rendering it highly stable and unreactive.
Unveiling Xenon’s Valence Electron Configuration
Xenon, positioned in Group 18 of the periodic table, possesses a unique atomic number of 54. This translates to an atomic structure with 54 electrons. Of these, the eight outermost electrons constitute xenon’s valence electrons. The configuration of these valence electrons, 6s²6p⁶, plays a crucial role in understanding xenon’s stability.
The Octet Rule and Xenon’s Stability
The octet rule dictates that atoms with eight valence electrons exhibit exceptional stability. Xenon, with its complete valence shell, fits perfectly into this rule. The filled s²p⁶ configuration creates a spherical electron cloud around the xenon atom, ensuring minimal interactions with other atoms. This electron configuration not only explains xenon’s inertness but also underpins its colorless, odorless, and non-flammable properties.
Applications of Xenon’s Unique Properties
Harnessing the unique properties of xenon has led to its diverse applications in various fields. Its inertness makes it an ideal component in incandescent light bulbs, preventing the filament from oxidizing and extending its lifespan. Xenon’s high density also finds use in medical imaging, particularly in CT scans, providing detailed cross-sectional images of the human body. Additionally, xenon’s anesthetic properties have been employed in surgical procedures, offering patients a safe and effective way to manage pain during surgery.
In conclusion, xenon’s valence electron configuration, in accordance with the octet rule, orchestrates its exceptional stability and inertness. This has not only shaped its position as a noble gas but also opened up a range of practical applications in various industries. Understanding the intricate interplay between valence electrons and the octet rule provides a deeper appreciation for the remarkable properties of elements like xenon.