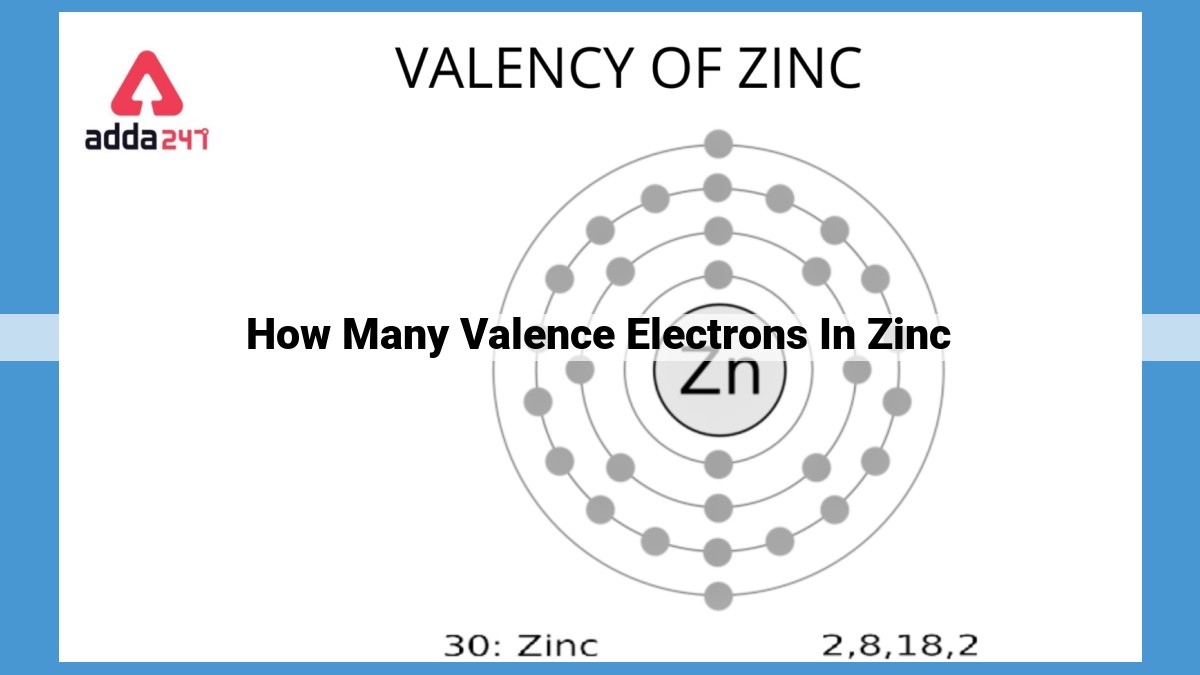
Zinc, a d-block element, possesses two valence electrons in its outermost shell. These electrons determine its chemical reactivity, as zinc readily loses them to achieve a stable noble gas configuration. Valence electrons influence the atomic radius, ionization energy, and electronegativity of zinc. The presence of valence electrons also explains its role in galvanization, where zinc protects metals from corrosion.
Valence Electrons: The Key Players in Chemistry’s Dance
In the fascinating realm of chemistry, where elements dance and rearrange to form countless substances, there’s a special group of electrons that hold the pivotal role: valence electrons. These are the electrons poised at the outermost shell of atoms, like eager performers waiting for their turn to waltz with other atoms. Their presence and behavior dictate the chemical reactions that shape our world.
Valence electrons, the gatekeepers of atoms, determine an element’s reactivity, bond formation capabilities, and many other fundamental properties. They are the architects of chemical reactions, orchestrating the interactions between different atoms to create new molecules. Understanding valence electrons is akin to deciphering the language of chemistry, unlocking the mysteries behind how matter transforms.
Unraveling the Enigma of Zinc’s Valence Electrons
In the vast symphony of elements, zinc stands out as a captivating d-block element, its atomic structure adorned with unfilled d-orbitals. This unique arrangement plays a pivotal role in zinc’s exceptional properties and its widespread use in various industrial applications.
Zinc’s electronic configuration, dictated by the Aufbau principle, reveals a fascinating dance of electrons. Its core electrons occupy the first three shells, while two valence electrons reside in the outermost shell, eagerly awaiting chemical interactions. These valence electrons, the gateway to zinc’s reactivity, determine the element’s penchant for forming bonds and shaping its distinctive properties.
Zinc’s valence electrons are the architects of its remarkable ability to prevent corrosion. In the process known as galvanization, zinc’s outer electrons act as sacrificial lambs, shielding other metals from the relentless assault of oxygen and water. This protective shield has made zinc an indispensable ally in the fight against rust and decay.
As we delve deeper into zinc’s atomic properties, we discover that its atomic radius is influenced by the valence electrons. A smaller number of valence electrons results in a more compact atom, while a larger number leads to a more diffuse atomic structure. Zinc’s valence electrons also play a crucial role in determining its ionization energy, the energy required to remove an electron from the atom’s grip. With two valence electrons, zinc exhibits a relatively low ionization energy, hinting at its eagerness to shed these electrons and achieve a more stable configuration.
Furthermore, zinc’s valence electrons influence its electronegativity, a measure of an atom’s ability to attract electrons. Zinc’s modest electronegativity reflects the relatively low affinity of its valence electrons for other electrons. This characteristic contributes to the formation of stable compounds where zinc readily shares its valence electrons, forging covalent bonds.
Zinc’s valence electrons, like skilled dancers, orchestrate the element’s chemical behavior, shaping its reactivity and influencing its atomic properties. By understanding the significance of these valence electrons, we gain a deeper appreciation for the remarkable versatility and industrial importance of this fascinating d-block element.
Valence Electrons: Zinc’s Chemical Key and Galvanization’s Savior
For a chemist, understanding valence electrons, those little players in an atom’s outermost shell, is like having the secret ingredient to a delicious dish. Valence electrons are the gatekeepers of chemical reactivity, determining how atoms cozy up with each other to form new substances.
Enter the world of zinc, a metal of intrigue with valence electrons that make it a shining star in the realm of chemical reactions. It’s a member of the d-block elements, boasting unfilled d-orbitals, like a teenager with a mischievous glint in their eye, ready to join the party.
But wait, there’s more! Zinc’s valence electrons don’t just dance by themselves. They play a crucial role in the magical process of galvanization, where zinc gallantly sacrifices its own electrons to protect other metals from the relentless onslaught of corrosion. It’s like zinc’s noble act of shielding its friends from the harsh realities of oxidation.
Unraveling Zinc’s Electronic Secrets
To truly grasp the power of zinc’s valence electrons, we need to dive into its electronic configuration, the blueprint of its atomic makeup. The Aufbau principle, like a wise architect, guides us through the orderly arrangement of electrons in zinc’s orbitals.
Imagine electrons as tiny planets orbiting the atom’s nucleus. The first two electrons reside in the 1s orbital, close to the nucleus like loyal bodyguards. The next two find their place in the 2s orbital, slightly farther away.
Then comes the exciting part: the 3d orbitals, which hold zinc’s valence electrons. The 3d orbital is like a futuristic spaceship, with room for up to 10 electrons. But zinc, our modest fellow, fills it with only two valence electrons.
Valence Electrons: The Movers and Shakers
These two valence electrons are zinc’s secret weapon, bestowing upon it the ability to form chemical bonds and interact with other atoms. They’re like the “social butterflies” of the atomic world, eager to dance with electrons from other elements.
The number of valence electrons also influences zinc’s chemical reactivity. With just two valence electrons, zinc readily loses them, striving to achieve the stability of a noble gas configuration. This noble pursuit makes zinc a reactive element, eager to participate in chemical reactions.
Atomic Properties: Valence Electrons’ Tangible Impact
Valence electrons don’t just influence chemical reactions; they also shape zinc’s atomic properties.
-
Atomic radius: Valence electrons, like a protective force field, determine the size of zinc’s atomic radius. The more valence electrons, the larger the atom’s radius.
-
Ionization energy: Valence electrons also affect how easily zinc can be ionized, or stripped of its electrons. Zinc’s low ionization energy means it can easily lose its valence electrons, making it a good reducing agent.
-
Electronegativity: Electronegativity measures an atom’s ability to attract electrons. Zinc’s low electronegativity indicates that its valence electrons are loosely held, making it a poor oxidizing agent.
Zinc’s valence electrons are the driving force behind its chemical properties, shaping its reactivity and influencing its atomic characteristics. From its role in galvanization, where it protects other metals from corrosion, to its ability to form bonds and create new substances, zinc’s valence electrons are essential to understanding its unique place in the world of chemistry.
The Significance of Valence Electrons: Understanding Zinc’s Chemical Properties
In the realm of chemistry, the concept of valence electrons holds paramount importance in unraveling the behavior of elements during chemical reactions. Valence electrons, the electrons residing in the outermost electron shell of an atom, dictate the element’s reactivity and play a pivotal role in determining its chemical properties.
Zinc, a fascinating element belonging to the d-block, stands as a testament to the significance of valence electrons. With its unfilled d-orbitals, zinc exhibits unique characteristics that make it a valuable material in various industrial applications. One such application is galvanization, where zinc’s protective prowess shields other metals from the ravages of corrosion.
To delve deeper into the intricacies of zinc’s chemistry, we embark on a journey to explore its electronic configuration. The Aufbau principle, a guiding principle in atomic physics, orchestrates the arrangement of electrons within an atom’s orbitals. According to this principle, electrons occupy the lowest energy orbitals first, gradually filling them until the atom reaches a stable configuration.
Zinc’s atomic number of 30 signifies that it possesses 30 electrons. Based on the Aufbau principle, we can determine its electronic configuration as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s²
From this configuration, it is evident that zinc possesses two valence electrons residing in the 4s orbital. These two valence electrons are pivotal in shaping zinc’s chemical reactivity. Elements with unpaired valence electrons tend to exhibit higher reactivity, and zinc is no exception. Its two unpaired valence electrons make it an eager participant in chemical reactions, enabling it to form stable compounds and participate in various chemical processes.
The number of valence electrons also influences other atomic properties of zinc. For instance, its atomic radius, the distance from the nucleus to the outermost electron shell, is determined by the shielding effect of the valence electrons. Additionally, the ionization energy, the energy required to remove an electron from an atom, is influenced by the number of valence electrons. Zinc’s relatively low ionization energy reflects the ease with which it can lose its valence electrons.
In the chemical realm, elements strive to attain a stable noble gas configuration, characterized by a full complement of valence electrons. Zinc, with its two valence electrons, can achieve this noble gas configuration by losing these two electrons. This loss results in the formation of a stable zinc ion with a +2 oxidation state.
In conclusion, zinc’s two valence electrons are the driving force behind its chemical properties. Understanding the significance of valence electrons enables us to unravel the mysteries of zinc’s reactivity and its diverse applications, from galvanization to its role in biological processes.
Zinc: Unraveling the Mysteries of Valence Electrons
Prologue
In the vast tapestry of chemistry, atoms dance an intricate ballet, each with its unique characteristics that shape the world around us. Among these atoms, zinc stands out as a captivating subject, its properties intimately intertwined with the enigmatic realm of valence electrons.
Act I: Enter the D-Block
Zinc resides within the d-block of the periodic table, a realm characterized by its unfilled d-orbitals. These orbitals, like miniature arenas, host electrons that play a pivotal role in zinc’s chemical behavior.
Act II: Galvanization, a Protective Shield
Zinc’s “d-block” status grants it a remarkable ability known as galvanization. This process involves coating other metals with a protective layer of zinc, shielding them from the ravages of corrosion. Zinc’s willingness to sacrifice its d-electrons in the service of protecting others is a testament to its selfless nature.
Act III: The Atomic Orchestra
To delve deeper into zinc’s secrets, we must understand the Aufbau principle, a conductor that orchestrates the arrangement of electrons within an atom. This principle dictates that electrons occupy orbitals in a systematic manner, starting with the lowest energy levels. Armed with this knowledge, we can now unravel the enigma of zinc’s electronic configuration.
Act IV: Unveiling Zinc’s Valence Electrons
At the forefront of zinc’s atomic stage, poised in the outermost shell, reside its two loyal valence electrons. These electrons, like intrepid explorers, venture beyond their atomic confines, eagerly participating in chemical reactions and determining zinc’s reactivity.
Act V: Atomic Symphony
The number of valence electrons in zinc not only governs its reactivity but also influences a host of atomic properties. These properties, like notes in a symphony, resonate with the presence of valence electrons, painting a vibrant portrait of zinc’s behavior.
Act VI: The Noble Gas Aspiration
Every atom strives for a stable, noble gas configuration, a state of electronic serenity. Zinc, in its relentless pursuit of stability, readily relinquishes two valence electrons, achieving the coveted noble gas configuration and forming a stable +2 oxidation state.
Epilogue
Zinc, with its two valiant valence electrons, stands as a shining example of the profound influence that these subatomic particles exert on the atomic realm. Through its d-block ancestry, electronic configuration, and captivating atomic properties, zinc weaves a captivating tale, revealing the intricate dance of valence electrons that shapes the very essence of matter.
Explaining that zinc has two valence electrons in its outermost shell.
Valence Electrons: The Powerhouse of Zinc
Meet zinc, a multifaceted element that plays a crucial role in our daily lives, from protecting metals to contributing to essential biological functions. But what makes zinc so remarkable? It all comes down to its valence electrons.
Zinc: A D-Block Star
Zinc resides in the d-block of the periodic table, a family of elements with unfilled d-orbitals. This unique feature gives zinc its distinct properties, including its role as a galvanizer. When applied to other metals, zinc forms a protective layer that shields them from corrosion.
Unveiling Zinc’s Electronic Configuration
Every atom has an electronic configuration, a map of its electrons distributed among energy levels. Zinc’s electronic configuration is determined by the Aufbau principle, which describes how electrons fill orbitals in a specific order. This arrangement reveals that zinc has two valence electrons in its outermost shell.
Valence Electrons: The Key to Reactivity
Valence electrons are the outermost electrons of an atom, and they dictate its chemical reactivity. Zinc’s two valence electrons make it a relatively reactive element, eager to participate in chemical reactions. This reactivity explains why zinc is found in various compounds, from batteries to pigments.
Atomic Properties: A Symphony of Valence Electrons
Valence electrons not only influence reactivity but also shape zinc’s atomic properties. They affect its atomic radius, the distance from the nucleus to the outermost electron shell. They also determine ionization energy, the energy required to remove an electron from the atom, and electronegativity, the atom’s ability to attract electrons.
Noble Gas Configuration: The Ultimate Goal
Atoms strive to achieve a stable configuration similar to noble gases, which have a full outermost shell. Zinc can achieve this stability by losing two valence electrons, resulting in a +2 oxidation state. This stable configuration explains why zinc often forms ionic bonds with other elements.
Zinc’s two valence electrons are the foundation of its remarkable properties. They govern its chemical reactivity, shape its atomic characteristics, and determine its ability to protect other metals. Understanding valence electrons is essential for comprehending the versatility of zinc and its vital role in our world.
Understanding Zinc’s Chemical Reactivity: The Role of Valence Electrons
Valence electrons are the electrons in an atom’s outermost shell, and they play a crucial role in determining an element’s chemical properties. In this blog post, we’ll explore the fascinating world of valence electrons, their significance in chemical reactions, and how they shape the reactivity of zinc, a versatile metal widely used in everyday applications.
Zinc: A d-Block Element
Zinc belongs to the d-block of the periodic table, characterized by unfilled d-orbitals. This unique electronic configuration gives zinc its distinctive properties, including its ability to form ionic bonds in galvanization, where zinc protects other metals from corrosion.
Zinc’s Electronic Configuration
The electronic configuration of zinc, determined by the Aufbau principle, is [Ar] 3d¹⁰ 4s². This means that zinc has two valence electrons in its outermost 4s orbital.
Valence Electrons and Reactivity
The number of valence electrons is a key factor in determining an element’s chemical reactivity. Zinc’s two valence electrons make it a relatively reactive metal. It readily loses these electrons to achieve a stable, noble gas configuration, forming a stable +2 oxidation state.
Atomic Properties Related to Valence Electrons
Valence electrons also influence various atomic properties, such as:
- Atomic radius: Valence electrons increase the electron cloud around the nucleus, increasing the atomic radius.
- Ionization energy: Valence electrons are relatively easy to remove, resulting in a low ionization energy.
- Electronegativity: Zinc has a low electronegativity, indicating its tendency to donate electrons and form positive ions.
Noble Gas Configuration
Chemical reactions are often driven by the desire of atoms to achieve a stable, noble gas configuration. Zinc loses two valence electrons to reach the electron configuration of the noble gas argon, [Ar]. This stable configuration makes zinc a useful reducing agent in various reactions.
Zinc’s two valence electrons play a pivotal role in shaping its chemical properties and reactivity. Its ability to form ionic bonds and achieve a stable noble gas configuration makes zinc an essential metal in diverse applications, from protecting against corrosion to providing a vital nutrient for human health. By understanding the importance of valence electrons, we gain a deeper appreciation for the fascinating world of chemistry and the remarkable properties of elements like zinc.
Unveiling the Secrets of Zinc: Valence Electrons and Beyond
In the realm of chemistry, valence electrons take center stage, dictating the reactivity and behavior of elements. Today’s exploration delves into the captivating world of zinc, a d-block element with a fascinating story to tell.
Zinc: The Protector of Metals
Zinc’s atomic number of 30 places it among the transition metals, known for their unfilled d-orbitals. These unfilled orbitals give zinc its unique properties, including its ability to form protective layers on other metals. This process, known as galvanization, is widely used to prevent corrosion and extend the lifespan of iron and steel structures.
Deciphering Zinc’s Electronic Blueprint
To unravel zinc’s chemical behavior, we must first understand its electronic configuration. The Aufbau principle guides us in this endeavor, dictating that electrons occupy specific orbitals based on their energy levels. Zinc’s electronic configuration is [Ar] 3d¹⁰ 4s², featuring two valence electrons in its outermost shell.
Valence Electrons: The Gateway to Reactivity
Zinc’s valence electrons, the outermost electrons, play a crucial role in its chemical reactivity. These electrons are easily lost or shared, making zinc a reactive metal. This reactivity manifests in its versatility, enabling it to form various compounds and participate in diverse chemical reactions.
Atomic Properties Influenced by Valence Electrons
Atomic radius: Valence electrons determine the size of an atom. The more valence electrons an element has, the larger its atomic radius. Zinc’s two valence electrons contribute to its relatively large atomic radius compared to other elements in the same period.
Zinc’s chemical properties stem from its valence electrons and the intricate interplay of its electronic configuration. As a d-block element, zinc’s unfilled d-orbitals and its ability to achieve a stable noble gas configuration further shape its behavior. Understanding the significance of valence electrons provides a gateway to comprehending the fascinating world of chemistry and the diverse elements that make up our universe.
Ionization energy: Explaining the relationship between valence electrons and the ionization energy of zinc.
Valence Electrons: The Key to Zinc’s Chemical Personality
Imagine a group of jovial partygoers, each with their own unique style and interests. In the realm of chemistry, these partygoers are called valence electrons, and they determine the chemical reactions that make up the world around us.
Amongst this vibrant crowd, zinc stands out as a member of the d-block elements, with unfilled d-orbitals like empty seats waiting to be filled. Zinc’s special talent lies in its ability to protect other metals from corrosion, a process known as galvanization. It’s like the protective bodyguard of the metal world.
To understand zinc’s chemical personality, we need to take a closer look at its electronic configuration. Just like the order in which guests arrive at a party, electrons fill atomic orbitals in a specific sequence, determined by the Aufbau principle. And just like each guest has their own unique energy level, electrons also have quantum numbers that describe their specific position and energy.
In the case of zinc, the party favors the outermost energy level, with two valence electrons eagerly waiting to mingle. These valence electrons are the chemical extroverts, interacting with other elements to form bonds and create new compounds. In fact, the number of valence electrons an element has is a major factor in determining its chemical reactivity. Zinc’s two valence electrons make it a relatively reactive element, eager to share the dance floor of chemical reactions.
But these valence electrons have more to do than just dance. They also influence zinc’s atomic properties, like the radius of its atomic bubble and the energy required to remove an electron (ionization energy). Zinc’s two valence electrons make it relatively small compared to other elements, and they also make it more difficult to remove an electron, giving it a higher ionization energy.
Finally, let’s talk about noble gas configuration, the ultimate goal of any chemical guest. Elements prefer to have the same number of valence electrons as a noble gas, which gives them a stable and unreactive state. Zinc aims for a noble gas configuration by losing two electrons, achieving a stable +2 oxidation state and leaving behind its lively valence electrons to play with other elements.
In conclusion, the number of valence electrons zinc has, two, is not just a number. It’s a key factor that determines its chemical properties, from its reactivity to its atomic size and ionization energy. Just like the lively partygoers who shape the atmosphere of a gathering, valence electrons are the heart and soul of an element’s chemical personality.
Valence Electrons and Zinc’s Chemical Properties: A D-Block Perspective
In the world of chemistry, electrons play a crucial role in determining how elements behave. Valence electrons, those found in the outermost shell of an atom, are particularly important as they dictate an element’s chemical reactivity. In this tale, we’ll explore the fascinating story of zinc, a d-block element with two valence electrons, and uncover its remarkable properties.
Zinc belongs to a special group of elements known as d-block elements, which possess unfilled d-orbitals. This unique electronic configuration grants zinc intriguing properties, including its ability to protect other metals from corrosion through a process called galvanization.
To delve deeper into zinc’s chemical behavior, let’s examine its electronic configuration. According to the Aufbau principle, electrons fill orbitals in a specific order, starting with the lowest energy level. Zinc has 30 electrons, and its electronic configuration is [Ar] 3d¹⁰ 4s². This means it has two valence electrons in its outermost 4s orbital.
These two valence electrons are the key to zinc’s chemical reactivity. They allow zinc to readily participate in chemical reactions, forming bonds with other atoms or molecules. This reactivity explains why zinc is often used in protective coatings, such as galvanization, where it sacrifices its own electrons to shield underlying metals from corrosion.
Beyond chemical reactivity, valence electrons also influence various atomic properties of zinc. The atomic radius, a measure of the size of an atom, is inversely proportional to the number of valence electrons. Zinc’s two valence electrons contribute to a relatively large atomic radius.
The ionization energy, the energy required to remove an electron from an atom, is directly proportional to the number of valence electrons. Zinc’s two valence electrons result in a relatively low ionization energy compared to other elements.
Finally, electronegativity, a measure of an atom’s ability to attract electrons, is inversely proportional to its atomic radius. Zinc’s large atomic radius, due to its two valence electrons, corresponds to a low electronegativity. This means zinc has a weak tendency to attract electrons, making it a relatively unreactive element.
In conclusion, zinc’s two valence electrons play a pivotal role in shaping its chemical properties. These electrons are responsible for zinc’s reactivity, its atomic radius, its ionization energy, and its electronegativity. Understanding these concepts provides a deeper appreciation of the fascinating world of chemistry and the elements that make up our universe.
Zinc’s Valence Electrons: Unlocking the Secrets of Chemical Reactions
In the realm of chemistry, valence electrons play a pivotal role, dictating the reactivity and behavior of elements. Among these elements, zinc stands out as a transition metal with a unique set of valence electrons that shape its chemical properties, making it an essential component in numerous industrial applications.
Zinc: The Protective Shield
Zinc belongs to the d-block of the periodic table, characterized by its unfilled d-orbitals. This distinct electronic configuration grants zinc the remarkable ability to protect other metals from corrosion through a process called galvanization. By coating iron or steel with zinc, a sacrificial layer is created that oxidizes preferentially, shielding the underlying metal from the damaging effects of oxygen and moisture.
Journey into Zinc’s Electronic Realm
Delving into the atomic structure of zinc, we encounter the Aufbau principle, a guiding principle that governs the arrangement of electrons in atomic orbitals. Zinc’s electronic configuration is [Ar] 3d¹⁰ 4s², indicating that it has two valence electrons in its outermost 4s orbital.
These valence electrons hold the key to zinc’s chemical reactivity. By participating in chemical reactions, zinc atoms strive to achieve a stable noble gas configuration, which has a full complement of eight electrons in the outermost shell. Zinc readily loses its two valence electrons to attain this stable state, forming a +2 oxidation state.
The Noble Gas Configuration: A Guiding Light
The noble gas configuration serves as a beacon of stability in the chemical world. Elements strive to mimic the electron configuration of noble gases, which possess filled outermost shells and exceptional inertness. Zinc’s two valence electrons are crucial for achieving this stable configuration, making it a highly reactive element.
Valence Electrons: Shaping Atomic Properties
The number of valence electrons profoundly influences the atomic properties of zinc.
- Atomic Radius: The presence of valence electrons in the outermost shell increases the atomic radius, as these electrons occupy orbitals that extend further from the nucleus.
- Ionization Energy: Removing valence electrons requires energy, resulting in a higher ionization energy for zinc.
- Electronegativity: Zinc’s electronegativity, a measure of its ability to attract electrons, is relatively low due to the presence of two valence electrons in a relatively large atomic radius.
Zinc’s two valence electrons are the architects of its chemical properties, dictating its reactivity and shaping its atomic characteristics. Understanding the role of valence electrons unravels the intricate tapestry of zinc’s behavior and illuminates its indispensable role in various industrial applications, from galvanization to batteries.
Unveiling the Valence Electrons of Zinc: A Gateway to Its Chemical Secrets
In the vast world of chemistry, the valence electrons, those electrons occupying the outermost energy level of an atom, play a pivotal role in determining an element’s chemical behavior. Zinc, a d-block element, is no exception, and its two valence electrons hold the key to its unique properties and reactivity.
Zinc: A Guardian against Corrosion
Zinc, with its unfilled d-orbitals, stands tall among the d-block elements. Its galvanization prowess is legendary, a process where zinc protects other metals from the ravages of corrosion. This ability stems from zinc’s willingness to sacrifice its own valence electrons to form a protective barrier, shielding the underlying metal from rust and decay.
Electronic Configuration: Unraveling Zinc’s Atomic Blueprint
To understand zinc’s valence electrons, we delve into its electronic configuration, a blueprint that reveals the arrangement of its electrons. Guided by the Aufbau principle, we build up zinc’s electron structure, filling its atomic orbitals one by one, starting with the lowest energy level. Zinc’s configuration, [Ar] 3d¹⁰ 4s², showcases its ten d-electrons and two valence electrons.
Valence Electrons: The Key to Zinc’s Reactivity
The two valence electrons, residing in the 4s orbital, are the gatekeepers of zinc’s chemical reactivity. Valence electrons are the ones that participate in chemical reactions, forming bonds with other atoms to create new substances. Zinc’s eagerness to lose these two electrons drives its high reactivity, making it a versatile player in a wide range of chemical processes.
Atomic Properties: A Reflection of Valence Electrons
Zinc’s valence electrons not only govern its reactivity but also influence its atomic properties. They play a role in determining zinc’s atomic radius, ionization energy, and electronegativity. A larger atomic radius indicates a more loosely held valence electron, while a higher ionization energy reflects the stronger attraction between the valence electrons and the nucleus. Electronegativity measures an atom’s ability to attract electrons, and zinc’s valence electrons contribute to its relatively low electronegativity.
Noble Gas Configuration: The Ultimate Goal
Chemical reactions often strive towards achieving a stable noble gas configuration, where atoms have the same electron configuration as helium or neon. For zinc, this means losing two valence electrons to attain a filled 3d¹⁰ configuration, resembling the stable electron arrangement of argon. This electron loss results in a stable +2 oxidation state, which zinc commonly adopts in its chemical compounds.
Zinc’s two valence electrons are the driving force behind its chemical behavior, determining its reactivity, atomic properties, and oxidation state. Understanding the role of valence electrons in zinc provides a deeper appreciation of its unique properties and its widespread use in galvanization and other industrial applications.
Zinc: Delving into the World of Valence Electrons
Zinc, a d-block element with unfilled d-orbitals, plays a crucial role in our daily lives. From galvanizing other metals to protect them from corrosion to being an essential nutrient for our bodies, zinc’s versatility stems from its unique electronic configuration.
Zinc’s valence electrons, the electrons in its outermost shell, are the key to understanding its chemical properties. With two valence electrons, zinc is eager to form chemical bonds to achieve a stable noble gas configuration.
The valence electrons of zinc influence its atomic properties. Its atomic radius, ionization energy, and electronegativity are all affected by the number and arrangement of these electrons. Zinc’s small atomic radius allows it to fit easily into various chemical structures, while its relatively low ionization energy makes it easy to remove these valence electrons.
As zinc forms chemical bonds, it typically loses its two valence electrons to achieve the stable +2 oxidation state. This loss of electrons creates a positive charge on the zinc ion, making it attractive to negatively charged species.
In conclusion, the two valence electrons of zinc are the driving force behind its chemical reactivity and unique properties. By understanding the role of valence electrons, we can better appreciate the diverse applications of this essential element in our world.
Valence Electrons: Unlocking the Secrets of Zinc’s Chemical Character
In the realm of chemistry, understanding valence electrons is akin to holding the key to unlocking the secrets of a substance’s behavior. These outermost electrons in an atom’s shell determine not only the atom’s reactivity but also its ability to form chemical bonds with other atoms.
One fascinating example is the element zinc, a d-block element that plays a vital role in myriad industrial and household applications. From protecting steel from rust to fueling our bodies with essential enzymes, zinc’s versatile properties stem from its unique electronic configuration.
Exploring Zinc’s Electronic Structure
Zinc, with an atomic number of 30, boasts 30 electrons arranged in energy levels or orbitals. According to the Aufbau principle, electrons fill the lowest energy orbitals first. In zinc’s case, its outermost energy level, known as the valence shell, contains two electrons.
These two valence electrons are the key players in determining zinc’s chemical properties. They dictate zinc’s tendency to lose two electrons to achieve a stable noble gas configuration, similar to helium or neon. This loss of electrons results in zinc’s characteristic +2 oxidation state.
The Significance of Valence Electrons
The number of valence electrons also influences several other atomic properties of zinc:
- Atomic radius: The presence of valence electrons in the outermost shell affects the overall size of the atom.
- Ionization energy: The energy required to remove a valence electron from an atom is directly related to the number of valence electrons. Zinc’s two valence electrons make it relatively easy to remove an electron, resulting in a lower ionization energy.
- Electronegativity: This measure of an atom’s ability to attract electrons is also influenced by the number of valence electrons. Zinc’s two valence electrons make it moderately electronegative.
The Versatility of Zinc
Zinc’s unique electronic configuration and atomic properties make it a versatile element with a wide range of applications:
- Galvanization: By coating iron or steel with a thin layer of zinc, we prevent rust and corrosion, extending the lifespan of these metals.
- Batteries: Zinc serves as the anode in many primary batteries, providing the chemical energy that powers electrical devices.
- Pharmaceuticals: Zinc is essential for many biological processes, and its compounds are used in various medications, such as cold remedies and anti-inflammatories.
Understanding the concept of valence electrons provides a window into the chemical behavior of zinc. With two valence electrons, zinc exhibits +2 oxidation state, forming stable compounds, and has a relatively low ionization energy. These properties, directly related to its electronic configuration, underpin zinc’s numerous industrial and biomedical applications, making it an indispensable element in our modern world.